GOLDSEA | ASIAMS.NET | ASIAN AMERICAN PERSONALITIES
BIOTECH GOLDENBOY
James Kuo, a 32-year-old biotech CEO, combined a medical degree with venture capital savvy to create some of today's hottest biotech companies.
by H Y Nahm
PAGE 1 OF 12
 t's the closest thing to magic in the business world. Start with a
concept, add a dash of biotechnology, top with a CEO. Wait a year or two and
spring the mix on a biotech-crazed investment world. Presto, you have a
company with a market valuation of $100 million or more.
t's the closest thing to magic in the business world. Start with a
concept, add a dash of biotechnology, top with a CEO. Wait a year or two and
spring the mix on a biotech-crazed investment world. Presto, you have a
company with a market valuation of $100 million or more.
| "You don't invest in the horse, you invest in the jockey." |
The key ingredient is the CEO.
"It's a commonplace in the venture capital community that you don't invest in the horse, you invest in the jockey," says James Kuo, one of the youngest jockeys around. He's only 32 and speaks in the precise, reassuring cadence of a man twice his age. The only difference is the energy level. There's a distinctly youthful turbocharge behind his conversational rifts.
"Most people consider the management to be the most important thing. Second would be the technology and third would be financing. If you have the management and technology, the financing would follow."
"I think investors look at the background of the individual and say. 'This is someone who's done things that are for real and has been successful doing it. He's going in a positive path.' When they see that, they're confident that the person is someone who's going to be successful and it's worth investing their money behind this person."
Case precisely in point, of course, is Kuo himself. He combined a Wharton MBA with a Penn MD to win entry into the biotech venture capital business. By the age of 27 he was directing a $380 million fund at America's leading biotech venture capital firm where he helped launch two of the world's newly minted biotech legends--Human Genome Sciences and Genetic Therapy Inc. Four-year-old HGS is already valued at $800 million and GTI was sold for $300 million.
Last March Kuo was tapped--by another 32-year-old goldenboy, chairman Steve Kanzer--to be CEO of an unfunded startup called Discovery Laboratories Inc (DLI). Kuo answered the call, promptly wrote a business plan and went on the road to raise $22 million in his first seven months. By the end of his first year, Kuo will have taken DLI through an IPO which will likely give it a $100-million-plus valuation. The big boost behind DLI's high-G-force launch into the biotech stratosphere came from the jockey, as Kuo points out, on whose sagaciously-stooped, nattily-tailored shoulders rests the task of raising bigtime capital.
A typical biotech startup burns cash during five to eight years of clinical trials, R&D and FDA approvals before it has a valuable drug. That means it can't realistically leave the pipe-dream stage unless it succeeds in wowing investors to the tune of $15-25 million. "One of the primary jobs as CEO is to present the company to investors throughout the world and inspire enough confidence that these are good opportunities and this is the proper management for taking it forward," Kuo says. "What the situation usually requires is an older, very experienced person from the pharmaceutical industry. It's not like investment banking, or entertainment or sports where youth is probably valued more than experience."
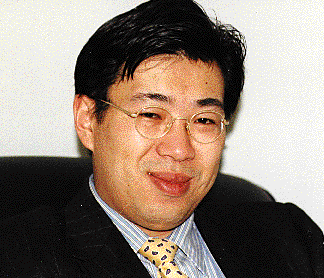
Kuo isn't shy about pointing out the obvious--rarely do you find someone 32-years-old heading up a company that's raised $22 million. To date, that's Kuo's crowning achievement.
Some small part of the credit goes to a fundamentally savvy business strategy that will likely become the industry business model in years to come. Right from the beginning, Kuo and Kanzer put their impatient fingertips on the most common cause of failure in biotech startups--technology that doesn't live up to its initial promise. In the conventional biotech business model a startup licenses a piece of breakthrough research from some university. It then spends three or more years and maybe $15-20 million to develop it into a lead compound that can withstand the arduous clinical trials process as a drug candidate for FDA approval. DLI's streamlined strategy is to skip that phase altogether by writing its business plan around three promising compounds that have already undergone extensive human testing.
The first is a drug candidate called SuperVent which is conceived as an aerosol for the treatment of cystic fibrosis (CF), the most common lethal genetic disease among caucasians affecting about 30,000 in the U.S. Down the road, DLI intends to put SuperVent through trials as a therapy for chronic bronchitis (CB), a far more widespread affliction also caused by degradation of breathing passages. What makes SuperVent a surer bet as a potential drug is that its active ingredient is tyloxapol, a compound that has been used for over 40 years by the U.S. drug industry as an emulsifying agent. That means its safety has already undergone FDA scrutiny, leaving only the hurdle of efficacy to be proven out through DLI's Phase I/II clinical trials which begin in mid-March. PAGE 2
|Page 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
CONTACT US
|
ADVERTISING INFO
© 1996-2013 Asian Media Group Inc
No part of the contents of this site may be reproduced without prior written permission.
